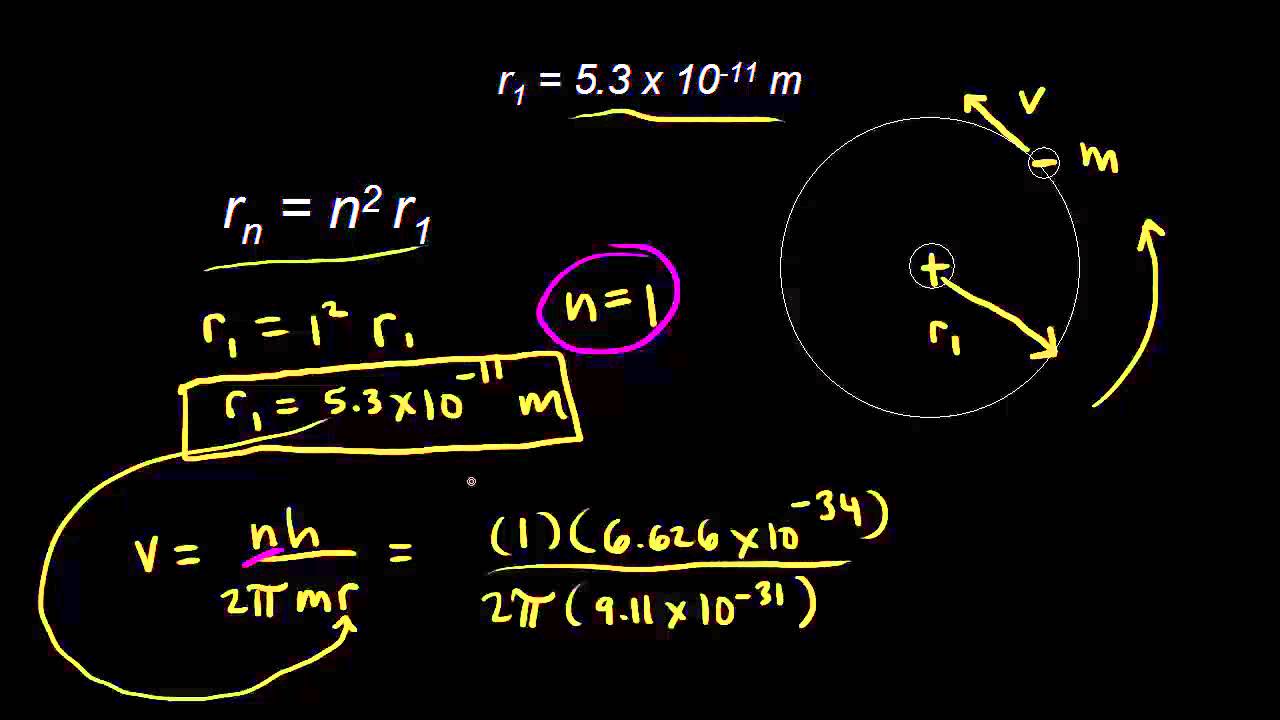OpenStax College Physics Solution, Chapter 30, Problem 13 (Problems & Exercises) | OpenStax College Physics Answers

The radius of the second Bohr orbit for hydrogen atom is: [Given: Planck's constant. h=6.6262×10^−34 Js; mass of electron = 9.1091×10^−31kg; charge of electron, e=1.60210×10^−19 C; permittivity of vacuum, (ε 0) =

Using Bohr's postulates of atomic model, derive the expression for radius of nth electron orbit. Hence, obtain the expression for Bohr's radius. | Snapsolve
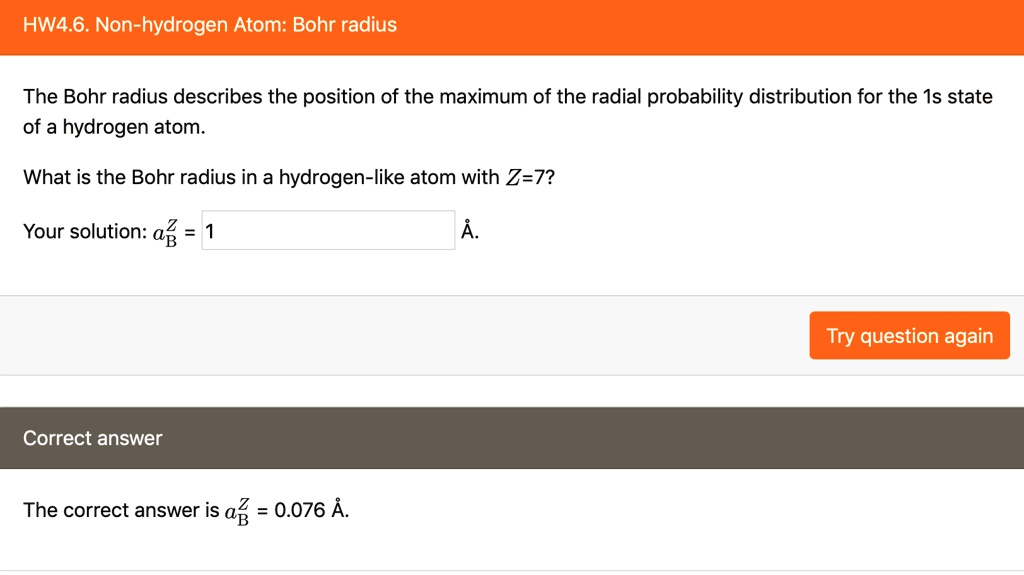
SOLVED:HW4.6. Non-hydrogen Atom: Bohr radius The Bohr radius describes the position of the maximum of the radial probability distribution for the Is state of a hydrogen atom_ What is the Bohr radius
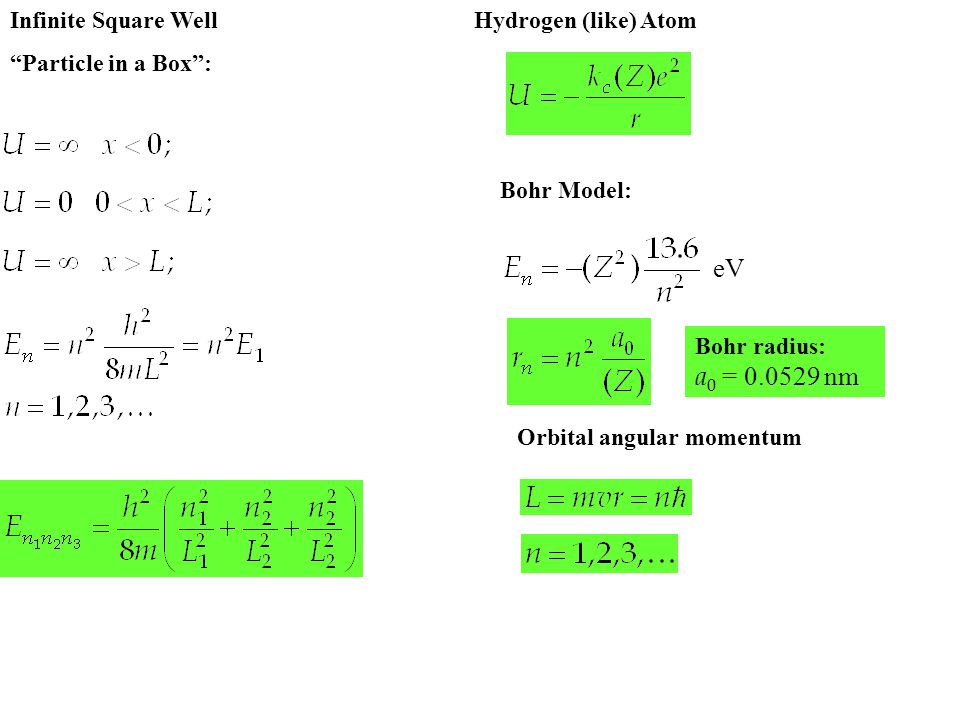
Infinite Square Well “Particle in a Box”: Hydrogen (like) Atom Bohr Model: eV Bohr radius: a 0 = nm Orbital angular momentum. - ppt download
What is the most possible radius (in PM) for an electron in the first orbit of a hydrogen atom? - Quora

MathType on Twitter: "The most probable distance between the nucleus and the electron in a hydrogen atom in its ground state is given by the Bohr Radius. This physical constant is named

1. The first Bohr's radius (for electron in hydrogen atom in the ground state): 2. The ground energy level in hydrogen atom: - ppt video online download

10. The value of Bohr's radius of hydrogen atom is : 0.529 x 10 cm 60.529 x 10-10 cm c) 0.529 x 10-12 cm d) 0.529 x 10-6 cm






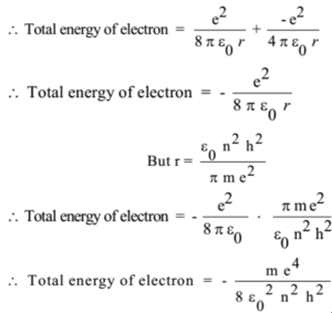


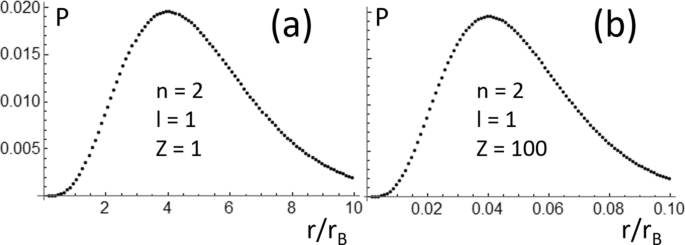
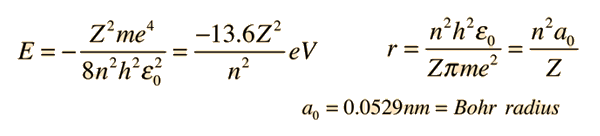
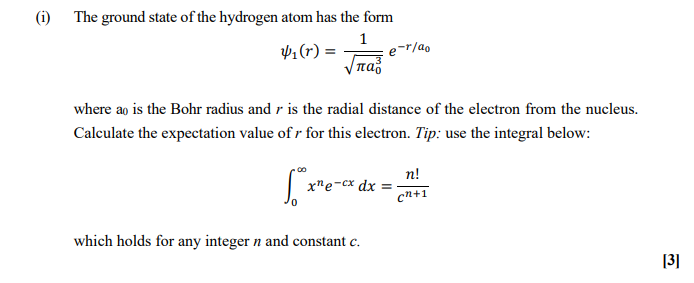
.PNG)